English 


The Yocon automatic immune cell culture workstation is a fully automatic immune cell culture equipment developed based on Yocon's high-performance version of NK bagged serum free culture medium and the automated piping system made of FEP material.
The overall equipment is highly intelligent. Once the cells are inoculated, no manual intervention is required, and the immune cells will be automatically harvested after 14 to 16 days of culturing.
It has the built-in functions of real-time cell density monitoring and data analysis, which can monitor cell growth and evaluate the culturing quality in real time, and can also produce high quality results for samples at different levels.
| Availability: | |
|---|---|
The Automatic Immune Cell Culture Workstation by Yocon is an advanced solution designed for fully automated immune cell culturing. Equipped with high-performance NK bagged serum-free culture medium and an automated piping system made of FEP material, this workstation minimizes manual intervention. Once cells are inoculated, the system autonomously handles the culturing process for 14 to 16 days, with automatic harvesting at the end.
Key features include real-time monitoring of cell density, data analysis, and an intelligent system that ensures consistent high-quality results for immune cell samples. The workstation’s simple user interface allows for easy management and observation of data, while the stable environment (37°C with 5% CO₂) optimizes cell growth and enhances the quality of cultured samples. The system's design ensures reproducibility and stability across different immune cell types, making it a reliable solution for advanced cell culturing needs.

Product Name: Automatic Immune Cell Culture Workstation
Culture Medium: High-performance NK bagged serum-free culture medium
Automation: Fully automated culturing process, no manual intervention required
Culturing Duration: 14 to 16 days
Cell Density Monitoring: Real-time non-contact density monitoring
Temperature: 37°C
CO₂ Concentration: 5%
Reagent Storage: Store at 2 - 8°C to maintain reagent stability
Data Management: Simple human-computer interaction interface for real-time data viewing and management
Harvesting: Automatic harvesting of immune cells after culturing period
Environment Control: Stable culturing environment for optimal cell growth
Applications: Suitable for various immune cell types, including NK cells from peripheral blood and umbilical cord blood
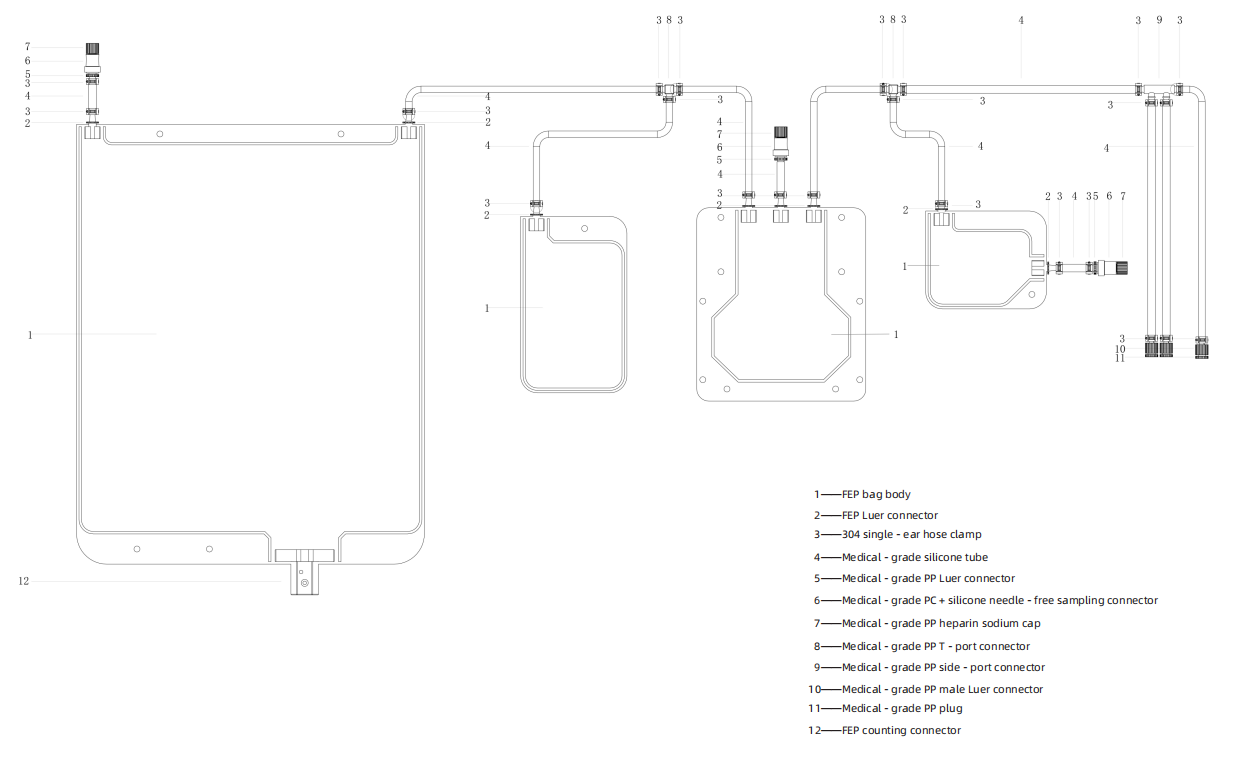
System Components: Automated piping system made of FEP material for precise reagent handling
User Interface: Intuitive operation interface for easy monitoring and control
The Automatic Immune Cell Culture Workstation automates the entire immune cell culturing process. Once cells are inoculated, no manual intervention is required, ensuring a hassle-free experience and reducing human error during culturing.
With a built-in non-contact cell density monitoring module, the workstation ensures precise, real-time tracking of immune cell growth, optimizing the culturing environment and maintaining consistent quality.
Set at 37°C with 5% CO₂, the workstation provides an ideal and stable environment for immune cell cultivation, ensuring high-quality results even for samples in different states.
The workstation features a simple, intuitive human-computer interaction interface, enabling easy management of data and monitoring of culturing progress, even for operators with minimal experience.
The reagent storage area ensures that the high-performance NK cell bagged culture medium remains stable at 2-8°C, preventing performance degradation from repeated pre-warming, thus preserving reagent quality.
This workstation supports automated culturing of various immune cell types, including NK cells from peripheral blood and umbilical cord blood, making it a versatile solution for different research and clinical needs.
At the end of the culturing period, the workstation automatically harvests the immune cells, further reducing labor and ensuring reliable and efficient results.
The Automatic Immune Cell Culture Workstation is ideal for immune cell research, particularly in the cultivation and study of NK cells. Its automated culturing process and real-time cell density monitoring ensure consistent and high-quality results, making it a reliable tool for research institutions focused on immunology and cell therapy.
The workstation is well-suited for clinical applications, including the automated culturing of NK cells from peripheral blood and umbilical cord blood. The ability to maintain a stable culturing environment at 37°C with 5% CO₂ is critical for producing high-quality immune cells for therapeutic use, such as in cancer immunotherapy.
In the biotech and pharmaceutical industries, the workstation enhances the efficiency and reliability of immune cell production. Its fully automated process minimizes human error and ensures reproducibility, crucial for large-scale manufacturing and clinical trials of immune-based treatments.
The workstation supports the automated cultivation of immune cells, which are essential for the production of cell-based therapies. Its ability to maintain optimal conditions for cell growth and provide automatic harvesting is a key advantage for companies developing cell therapies for a variety of diseases, including cancers and autoimmune disorders.
In stem cell research, the Automatic Immune Cell Culture Workstation can be utilized for cultivating immune cells alongside stem cells, facilitating studies on cell interactions, differentiation, and therapeutic potentials, all while ensuring accurate monitoring and harvesting of cultured cells.
For personalized medicine, the workstation helps produce immune cells tailored to individual patients. The automated system ensures consistent quality and reproducibility, crucial for creating patient-specific cell therapies and treatments.
Automated immune cell culturing ensures consistent results by eliminating variability from manual processes, offering reliable and precise cell growth monitoring.
Automation reduces the need for continuous monitoring and manual intervention, leading to significant savings in time and resources.
With real-time cell density monitoring and data analysis, the system ensures optimal culturing conditions, resulting in high-quality immune cell cultures.
The automated system eliminates the potential for human error, ensuring more accurate and reproducible outcomes during immune cell harvesting.
By streamlining the culturing process, automation reduces labor costs and enhances overall cost-effectiveness in immune cell research and clinical applications.
Automated culturing systems can handle larger sample volumes, making them scalable and adaptable for various research and clinical needs.

Cell Expansion: Achieves over 200 times cell expansion with a positive rate of over 85% after 14 to 16 days of culturing.
Versatile Application: Suitable for culturing both peripheral blood and umbilical cord blood, with both fresh and frozen samples.
Simplified Culturing: Induced by pure factors without trophoblast cells, ensuring uniform cell states and stable results when following the recommended medium replenishment procedures.
FDA Compliant: Can be combined with research-grade basal medium (NC0102) or chemically defined basal medium (NC0102.H), both FDA DMF filed (Filing Number: 37874).

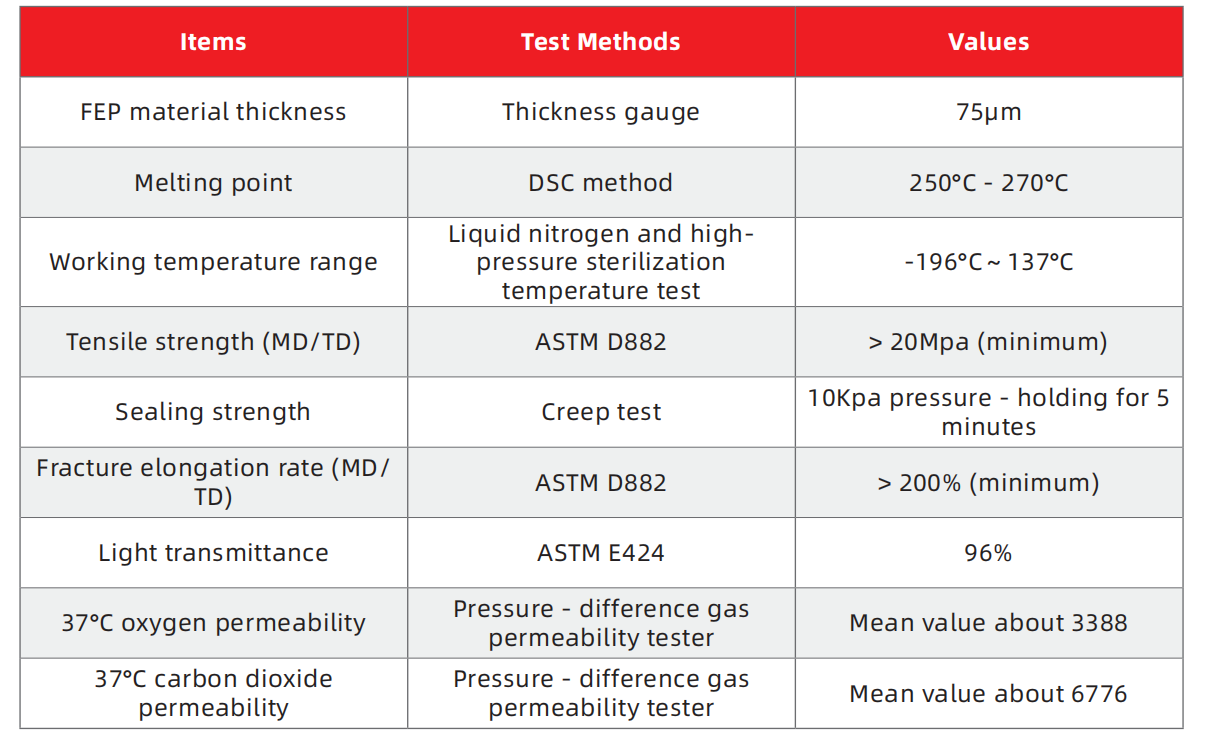
Enhanced Oxygen and Vapor Barrier: FEP (Fluorinated Ethylene Propylene Copolymer) offers superior oxygen permeability and water vapor barrier properties, resulting in higher cell counts under the same culture volume compared to EVA.
Light Transmittance: FEP’s over 95% light transmittance enables intuitive and real-time observation of cell conditions, facilitating non-contact cell density monitoring.
Biocompatibility and Durability: FEP is 100% inert and free from additives, ensuring excellent biocompatibility. It withstands extreme temperatures from -196°C to 137°C, meeting USP Class VI requirements.
Leak-Proof Technology: The cell culture bags are made with laser welding technology, minimizing the risk of liquid leakage and ensuring secure seals.
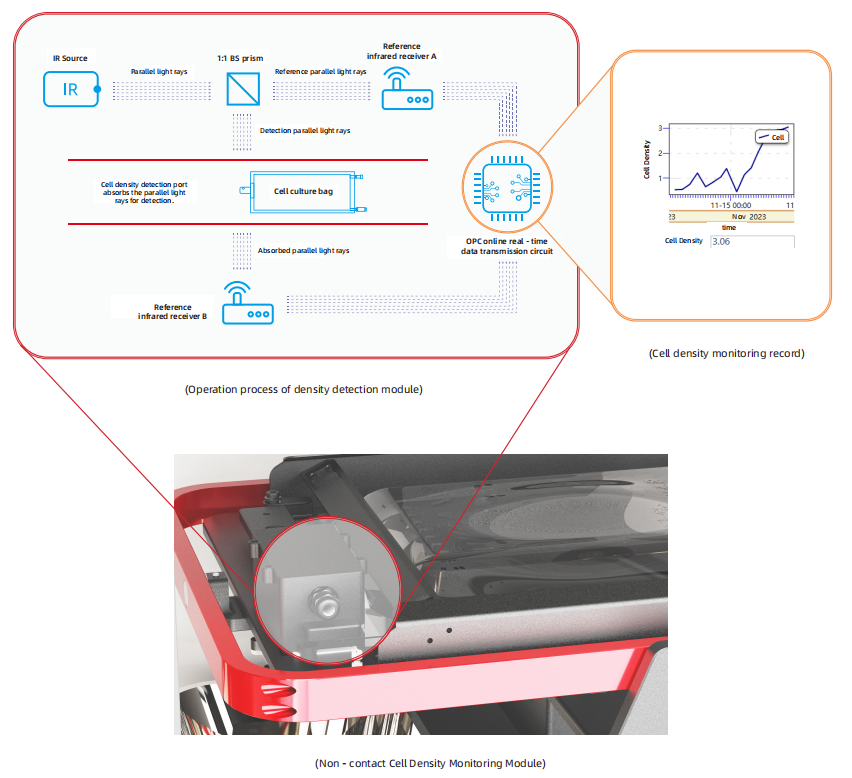
Infrared Monitoring: The system uses infrared light beams to monitor cell density without contact.
Accurate Measurement: The optical signal-processing chip processes the refracted infrared light signals passing through the cells, providing accurate non-contact cell density records.
Culturing Level Optimization: This technology allows for precise judgments of the culturing levels and automatic adjustment of fluid replacement, ensuring optimal culturing conditions for different sample types.


Note: Cases 1 - 5 are automated cultures of peripheral blood NK samples; Cases 6 and 7 are automated cultures of umbilical cord blood NK samples. The last three are customer cases, all of which are automated cultures of peripheral blood NK samples. Among them, customer cases two and three did not provide flow cytometry test diagrams.
The Automatic Immune Cell Culture Workstation by Yocon is a fully automated system designed to streamline the immune cell culturing process. By utilizing high-performance NK bagged serum-free culture medium and a sophisticated automated piping system, the workstation ensures precise, hands-off culturing of immune cells for 14 to 16 days. Real-time monitoring and automatic harvesting guarantee optimal results with minimal human intervention. This system is ideal for research institutions, clinical applications, biotech companies, and personalized medicine.
With its user-friendly interface, stable culturing environment, and scalable functionality, the workstation offers a reliable, cost-effective solution for high-quality immune cell production.
Ready to enhance your cell culturing process? Contact us today to learn more!
The Automatic Immune Cell Culture Workstation by Yocon is an advanced solution designed for fully automated immune cell culturing. Equipped with high-performance NK bagged serum-free culture medium and an automated piping system made of FEP material, this workstation minimizes manual intervention. Once cells are inoculated, the system autonomously handles the culturing process for 14 to 16 days, with automatic harvesting at the end.
Key features include real-time monitoring of cell density, data analysis, and an intelligent system that ensures consistent high-quality results for immune cell samples. The workstation’s simple user interface allows for easy management and observation of data, while the stable environment (37°C with 5% CO₂) optimizes cell growth and enhances the quality of cultured samples. The system's design ensures reproducibility and stability across different immune cell types, making it a reliable solution for advanced cell culturing needs.

Product Name: Automatic Immune Cell Culture Workstation
Culture Medium: High-performance NK bagged serum-free culture medium
Automation: Fully automated culturing process, no manual intervention required
Culturing Duration: 14 to 16 days
Cell Density Monitoring: Real-time non-contact density monitoring
Temperature: 37°C
CO₂ Concentration: 5%
Reagent Storage: Store at 2 - 8°C to maintain reagent stability
Data Management: Simple human-computer interaction interface for real-time data viewing and management
Harvesting: Automatic harvesting of immune cells after culturing period
Environment Control: Stable culturing environment for optimal cell growth
Applications: Suitable for various immune cell types, including NK cells from peripheral blood and umbilical cord blood
System Components: Automated piping system made of FEP material for precise reagent handling
User Interface: Intuitive operation interface for easy monitoring and control
The Automatic Immune Cell Culture Workstation automates the entire immune cell culturing process. Once cells are inoculated, no manual intervention is required, ensuring a hassle-free experience and reducing human error during culturing.
With a built-in non-contact cell density monitoring module, the workstation ensures precise, real-time tracking of immune cell growth, optimizing the culturing environment and maintaining consistent quality.
Set at 37°C with 5% CO₂, the workstation provides an ideal and stable environment for immune cell cultivation, ensuring high-quality results even for samples in different states.
The workstation features a simple, intuitive human-computer interaction interface, enabling easy management of data and monitoring of culturing progress, even for operators with minimal experience.
The reagent storage area ensures that the high-performance NK cell bagged culture medium remains stable at 2-8°C, preventing performance degradation from repeated pre-warming, thus preserving reagent quality.
This workstation supports automated culturing of various immune cell types, including NK cells from peripheral blood and umbilical cord blood, making it a versatile solution for different research and clinical needs.
At the end of the culturing period, the workstation automatically harvests the immune cells, further reducing labor and ensuring reliable and efficient results.
The Automatic Immune Cell Culture Workstation is ideal for immune cell research, particularly in the cultivation and study of NK cells. Its automated culturing process and real-time cell density monitoring ensure consistent and high-quality results, making it a reliable tool for research institutions focused on immunology and cell therapy.
The workstation is well-suited for clinical applications, including the automated culturing of NK cells from peripheral blood and umbilical cord blood. The ability to maintain a stable culturing environment at 37°C with 5% CO₂ is critical for producing high-quality immune cells for therapeutic use, such as in cancer immunotherapy.
In the biotech and pharmaceutical industries, the workstation enhances the efficiency and reliability of immune cell production. Its fully automated process minimizes human error and ensures reproducibility, crucial for large-scale manufacturing and clinical trials of immune-based treatments.
The workstation supports the automated cultivation of immune cells, which are essential for the production of cell-based therapies. Its ability to maintain optimal conditions for cell growth and provide automatic harvesting is a key advantage for companies developing cell therapies for a variety of diseases, including cancers and autoimmune disorders.
In stem cell research, the Automatic Immune Cell Culture Workstation can be utilized for cultivating immune cells alongside stem cells, facilitating studies on cell interactions, differentiation, and therapeutic potentials, all while ensuring accurate monitoring and harvesting of cultured cells.
For personalized medicine, the workstation helps produce immune cells tailored to individual patients. The automated system ensures consistent quality and reproducibility, crucial for creating patient-specific cell therapies and treatments.
Automated immune cell culturing ensures consistent results by eliminating variability from manual processes, offering reliable and precise cell growth monitoring.
Automation reduces the need for continuous monitoring and manual intervention, leading to significant savings in time and resources.
With real-time cell density monitoring and data analysis, the system ensures optimal culturing conditions, resulting in high-quality immune cell cultures.
The automated system eliminates the potential for human error, ensuring more accurate and reproducible outcomes during immune cell harvesting.
By streamlining the culturing process, automation reduces labor costs and enhances overall cost-effectiveness in immune cell research and clinical applications.
Automated culturing systems can handle larger sample volumes, making them scalable and adaptable for various research and clinical needs.

Cell Expansion: Achieves over 200 times cell expansion with a positive rate of over 85% after 14 to 16 days of culturing.
Versatile Application: Suitable for culturing both peripheral blood and umbilical cord blood, with both fresh and frozen samples.
Simplified Culturing: Induced by pure factors without trophoblast cells, ensuring uniform cell states and stable results when following the recommended medium replenishment procedures.
FDA Compliant: Can be combined with research-grade basal medium (NC0102) or chemically defined basal medium (NC0102.H), both FDA DMF filed (Filing Number: 37874).

Enhanced Oxygen and Vapor Barrier: FEP (Fluorinated Ethylene Propylene Copolymer) offers superior oxygen permeability and water vapor barrier properties, resulting in higher cell counts under the same culture volume compared to EVA.
Light Transmittance: FEP’s over 95% light transmittance enables intuitive and real-time observation of cell conditions, facilitating non-contact cell density monitoring.
Biocompatibility and Durability: FEP is 100% inert and free from additives, ensuring excellent biocompatibility. It withstands extreme temperatures from -196°C to 137°C, meeting USP Class VI requirements.
Leak-Proof Technology: The cell culture bags are made with laser welding technology, minimizing the risk of liquid leakage and ensuring secure seals.


Infrared Monitoring: The system uses infrared light beams to monitor cell density without contact.
Accurate Measurement: The optical signal-processing chip processes the refracted infrared light signals passing through the cells, providing accurate non-contact cell density records.
Culturing Level Optimization: This technology allows for precise judgments of the culturing levels and automatic adjustment of fluid replacement, ensuring optimal culturing conditions for different sample types.



Note: Cases 1 - 5 are automated cultures of peripheral blood NK samples; Cases 6 and 7 are automated cultures of umbilical cord blood NK samples. The last three are customer cases, all of which are automated cultures of peripheral blood NK samples. Among them, customer cases two and three did not provide flow cytometry test diagrams.
The Automatic Immune Cell Culture Workstation by Yocon is a fully automated system designed to streamline the immune cell culturing process. By utilizing high-performance NK bagged serum-free culture medium and a sophisticated automated piping system, the workstation ensures precise, hands-off culturing of immune cells for 14 to 16 days. Real-time monitoring and automatic harvesting guarantee optimal results with minimal human intervention. This system is ideal for research institutions, clinical applications, biotech companies, and personalized medicine.
With its user-friendly interface, stable culturing environment, and scalable functionality, the workstation offers a reliable, cost-effective solution for high-quality immune cell production.
Ready to enhance your cell culturing process? Contact us today to learn more!