English 


| Availability: | |
|---|---|
High Yield Efficiency
The primary advantage of our kit is its unparalleled ability to consistently achieve high yields of functional NK cells from umbilical blood, which is crucial for successful clinical and research applications.
Tailored Adaptability
Its unique modularity offers unmatched flexibility, enabling you to precisely tailor the culture process to specific blood volumes. This approach significantly minimizes waste and maximizes efficiency, optimizing your resource utilization.
Robust Cell Functionality
Through a carefully formulated culture environment, the kit ensures robust cell expansion while maintaining the vital functionality of the NK cells, pushing the boundaries of what's achievable in immune cell research and therapy
High Yield Efficiency
The primary advantage of our kit is its unparalleled ability to consistently achieve high yields of functional NK cells from umbilical blood, which is crucial for successful clinical and research applications.
Tailored Adaptability
Its unique modularity offers unmatched flexibility, enabling you to precisely tailor the culture process to specific blood volumes. This approach significantly minimizes waste and maximizes efficiency, optimizing your resource utilization.
Robust Cell Functionality
Through a carefully formulated culture environment, the kit ensures robust cell expansion while maintaining the vital functionality of the NK cells, pushing the boundaries of what's achievable in immune cell research and therapy

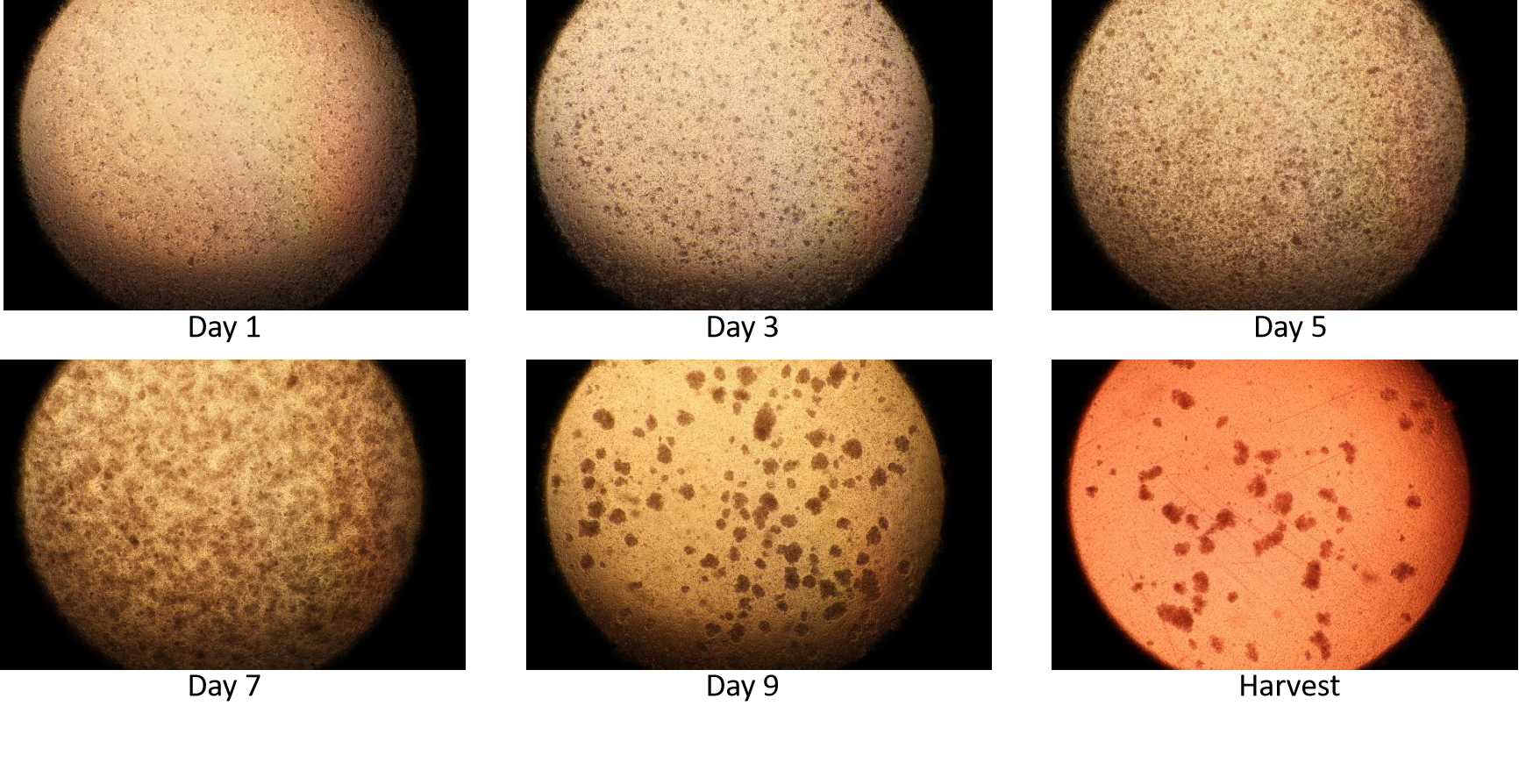
NK Cell Expansion Case
| | | | | | | | | | |
|---|---|---|---|---|---|---|---|---|---|
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |

NK Cell Expansion Case
| | | | | | | | | | |
|---|---|---|---|---|---|---|---|---|---|
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |

Product information
NK Large-Scale Culture Kit —— Standard Components
| | | | | |
|---|---|---|---|---|
| | | | | |
| | | | | |
| | | YC00A: Used for coating culture flasks during initial culture | 1 vial, 500μL/vial | |
YC00B: Added during initial culture | 1 vial, 500μL/vial | |||
YC00C: Added to activation culture medium | 1 vial, 200μL/vial | |||
YC005: Added to expansion culture medium | 3 vials | |||
Gentamicin: Added to culture medium for use | 1 vial, 300μL/vial | |||
| | | | | |
NK Large-Scale Culture Kit —— Standard Components
| | | | | |
|---|---|---|---|---|
| | | | | |
| | | | | |
| | | YC00A: For coating culture bottles during initial culture | 1 vial, 250μL/vial | |
YC00B: For addition during initial culture | 1 vial, 250μL/vial | |||
| YC00C: Addition to activation medium | 1 vial, 100μL/vial | |||
| YC005: Addition to expansion medium | 1 vial | |||
Gentamicin: For addition to culture medium | | |||
| | | | | |
| | | | | |
The kit's modularity allows for precise control over the culture process. Begin with the Standard Components for approximately 80g of umbilical blood or 50mL of pure umbilical blood. For every additional 20g of umbilical blood beyond this, integrate an Expansion Component to ensure optimal nutrient and factor availability, enabling proportionate cell growth and achieving your target cell counts.

Product information
NK Large-Scale Culture Kit —— Standard Components
| | | | | |
|---|---|---|---|---|
| | | | | |
| | | | | |
| | | YC00A: Used for coating culture flasks during initial culture | 1 vial, 500μL/vial | |
YC00B: Added during initial culture | 1 vial, 500μL/vial | |||
YC00C: Added to activation culture medium | 1 vial, 200μL/vial | |||
YC005: Added to expansion culture medium | 3 vials | |||
Gentamicin: Added to culture medium for use | 1 vial, 300μL/vial | |||
| | | | | |
NK Large-Scale Culture Kit —— Standard Components
| | | | | |
|---|---|---|---|---|
| | | | | |
| | | | | |
| | | YC00A: For coating culture bottles during initial culture | 1 vial, 250μL/vial | |
YC00B: For addition during initial culture | 1 vial, 250μL/vial | |||
| YC00C: Addition to activation medium | 1 vial, 100μL/vial | |||
| YC005: Addition to expansion medium | 1 vial | |||
Gentamicin: For addition to culture medium | | |||
| | | | | |
| | | | | |
The kit's modularity allows for precise control over the culture process. Begin with the Standard Components for approximately 80g of umbilical blood or 50mL of pure umbilical blood. For every additional 20g of umbilical blood beyond this, integrate an Expansion Component to ensure optimal nutrient and factor availability, enabling proportionate cell growth and achieving your target cell counts.

Samples transported within 8 hours with anticoagulant ≤28% and samples transported 8–24 hours with anticoagulant ≤25% can be cultured using autologous plasma.
For samples with incompatible anticoagulant ratios, validated commercial blood substitutes or human AB serum can replace autologous plasma.
Samples with shorter transport times and lower anticoagulant ratios yield higher-quality MNCs.
After red blood cell lysis, the red blood cell ratio should be <50%.
For cryopreserved MNCs: Repair cells in immunorepair medium before culture. Post-repair spontaneous apoptosis should be ~20%.
Not recommended. Adjusting the inoculum may cause mismatched factor quantities in the kit, compromising final cell yield or purity.
Seed 1 T75 flask (6E7 cells) and supplement with 1–2 T25 flasks to utilize the remaining cells.
Yes. Preferably use Youkang single-cell cryopreservation medium or other validated immunocyte cryopreservation media. Ensure post-thaw cell viability ≥90%. Due to cryopreservation-induced damage, a portion of cells may undergo apoptosis after inoculation. We recommend "repair before culture": Incubate thawed cells in immunorepair medium to restore viability before proceeding.
Days 3 and 5: Follow the manual strictly for feeding and plasma supplementation.
After Day 7: Adjust feeding based on cell status (e.g., reduce volume if metabolic activity slows).
Most qualified samples can reach 6L; some may scale to 12L. Key indicator: If cell numbers double within 2–3 days post-feeding, the culture retains strong expansion capacity—proceed with further feeding.
Samples transported within 8 hours with anticoagulant ≤28% and samples transported 8–24 hours with anticoagulant ≤25% can be cultured using autologous plasma.
For samples with incompatible anticoagulant ratios, validated commercial blood substitutes or human AB serum can replace autologous plasma.
Samples with shorter transport times and lower anticoagulant ratios yield higher-quality MNCs.
After red blood cell lysis, the red blood cell ratio should be <50%.
For cryopreserved MNCs: Repair cells in immunorepair medium before culture. Post-repair spontaneous apoptosis should be ~20%.
Not recommended. Adjusting the inoculum may cause mismatched factor quantities in the kit, compromising final cell yield or purity.
Seed 1 T75 flask (6E7 cells) and supplement with 1–2 T25 flasks to utilize the remaining cells.
Yes. Preferably use Youkang single-cell cryopreservation medium or other validated immunocyte cryopreservation media. Ensure post-thaw cell viability ≥90%. Due to cryopreservation-induced damage, a portion of cells may undergo apoptosis after inoculation. We recommend "repair before culture": Incubate thawed cells in immunorepair medium to restore viability before proceeding.
Days 3 and 5: Follow the manual strictly for feeding and plasma supplementation.
After Day 7: Adjust feeding based on cell status (e.g., reduce volume if metabolic activity slows).
Most qualified samples can reach 6L; some may scale to 12L. Key indicator: If cell numbers double within 2–3 days post-feeding, the culture retains strong expansion capacity—proceed with further feeding.