English 


| Availability: | |
|---|---|
YOCON's Umbilical Cord Mesenchymal Stem Cell Serum-Free Medium represents a significant leap forward in cell culture technology. Designed with the demanding needs of stem cell research and therapeutic applications in mind, this medium provides an optimal, defined environment for MSC proliferation. Its unique formulation eliminates the variability and safety concerns associated with serum-containing media, offering a consistent and reliable solution for high-quality MSC production. Our dedication to innovation and quality ensures that researchers and developers can achieve reproducible and clinically relevant outcomes.

Property | Detail |
Product Name | Umbilical Cord Mesenchymal Stem Cell Serum-Free Medium |
Primary Use | Primary isolation and expansion of MSCs from various tissue sources |
Components | Pure factor system, completely serum-free |
Animal-Derived | Free |
Human-Derived | Free |
Regulatory Status | US FDA Class II Medical Device Registration (510(K): K232543) |
Yield (Umbilical Cord) | >2.4 x 10⁷ cells from 20 cm normal umbilical cord (primary isolation) |
Passage Stability | Maintains stable cell characteristics up to P20 |
The difference between serum-free and serum-free culture media

YOCON's Umbilical Cord Mesenchymal Stem Cell Serum-Free Medium represents a significant leap forward in cell culture technology. Designed with the demanding needs of stem cell research and therapeutic applications in mind, this medium provides an optimal, defined environment for MSC proliferation. Its unique formulation eliminates the variability and safety concerns associated with serum-containing media, offering a consistent and reliable solution for high-quality MSC production. Our dedication to innovation and quality ensures that researchers and developers can achieve reproducible and clinically relevant outcomes.

Property | Detail |
Product Name | Umbilical Cord Mesenchymal Stem Cell Serum-Free Medium |
Primary Use | Primary isolation and expansion of MSCs from various tissue sources |
Components | Pure factor system, completely serum-free |
Animal-Derived | Free |
Human-Derived | Free |
Regulatory Status | US FDA Class II Medical Device Registration (510(K): K232543) |
Yield (Umbilical Cord) | >2.4 x 10⁷ cells from 20 cm normal umbilical cord (primary isolation) |
Passage Stability | Maintains stable cell characteristics up to P20 |
The difference between serum-free and serum-free culture media

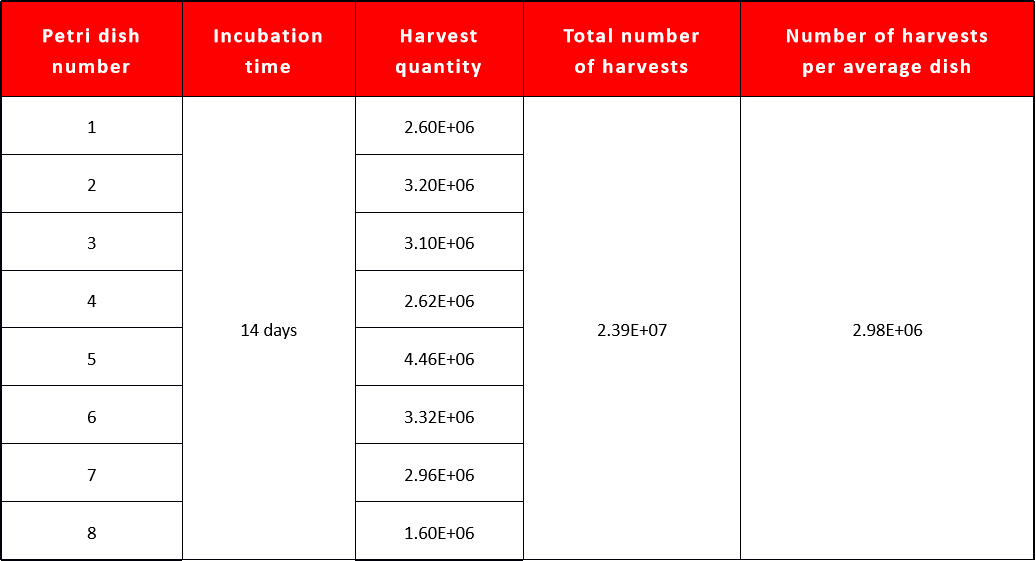
Approximately 2.4 × 10^7 primary cells can be obtained from a fresh 20cm umbilical cord.
Primary cells begin to migrate out within 5-9 days.
Primary cells can be harvested within 12-14 days.
Data on primordial cell isolation
A single umbilical cord can yield approximately 16cm of usable tissue, with each 150mm culture dish requiring about 2cm of cord. This allows for the cultivation of up to 8 dishes. On average, 150 such dishes (equivalent to a T175 culture flask) can produce between 1E6 and 3E6 primary cells, with each cord yielding 8E6-2.4E7 cells.
Primary cells isolated from the umbilical cord or cells from the established seed bank can be subcultured and stably passaged up to 20 generations.
Total yield of P3 cells: 6480 × 10^7 cells.
Total yield of P10 cells: 58032 × 10^13 cells.
Total yield of P20 cells: 424351 × 10^20 cells.
Data of Continuous Subculturing
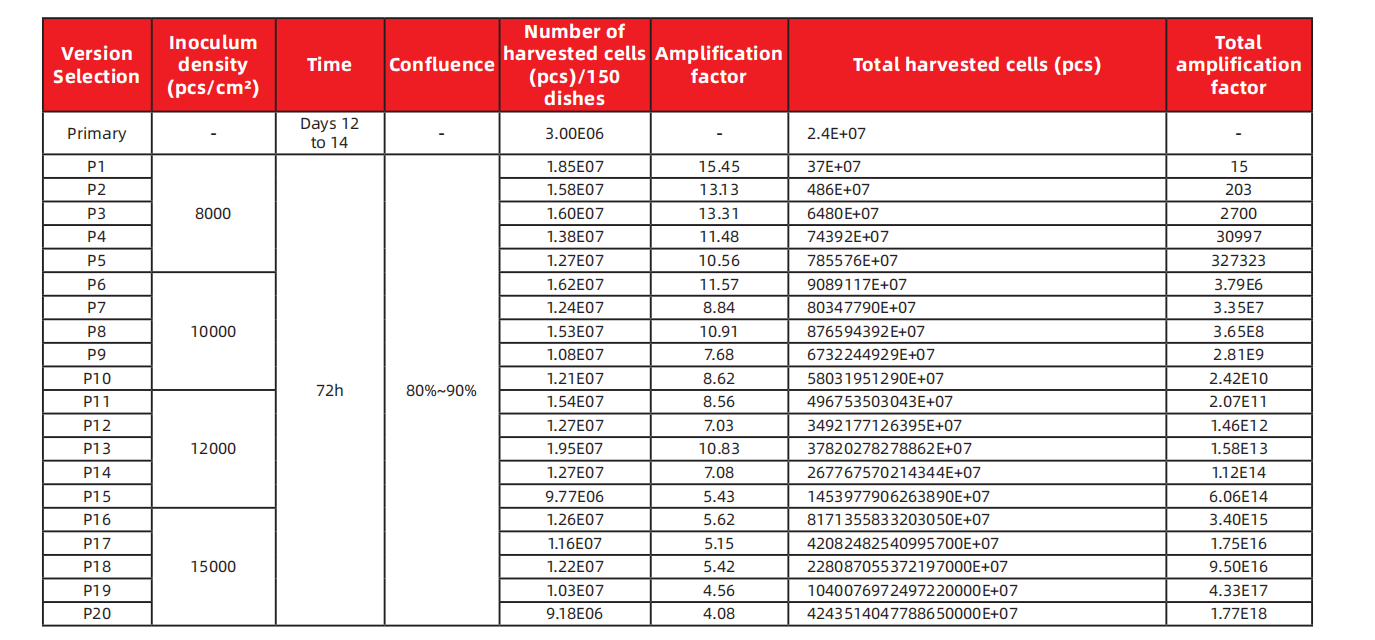
*The above data was obtained from serum-free medium and stem cell mild digestive enzymes by Yocon Biology primary separation technique. Different umbilical cord samples and different separation techniques may lead to significant differences in results.
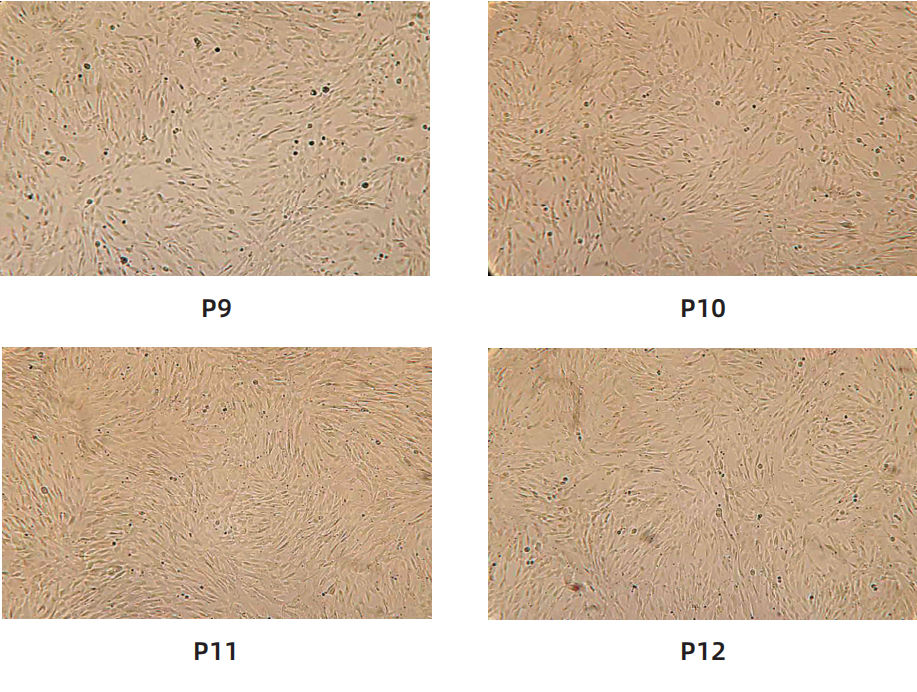
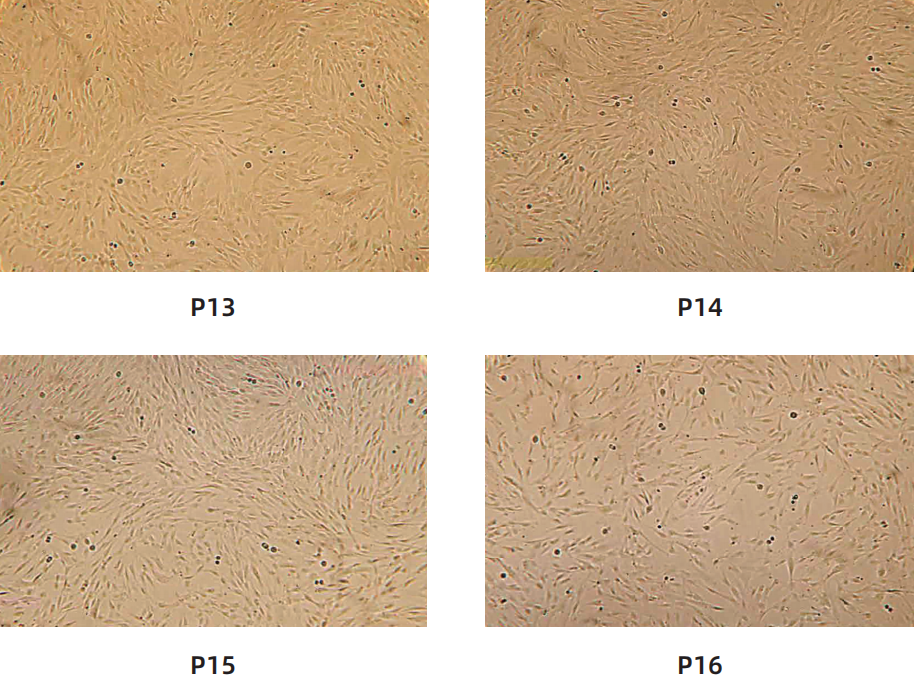
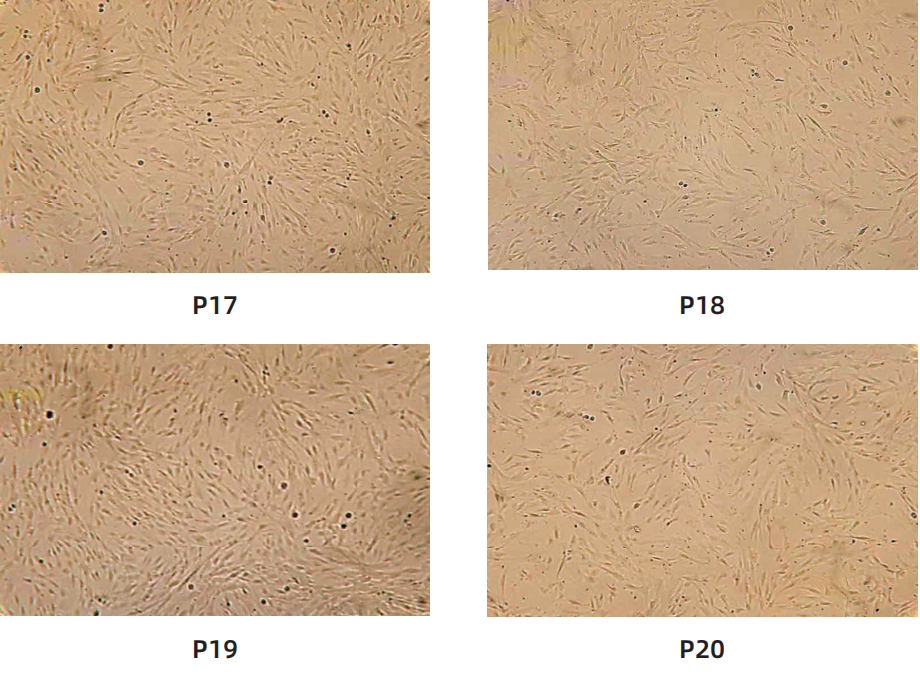
Cell morphology
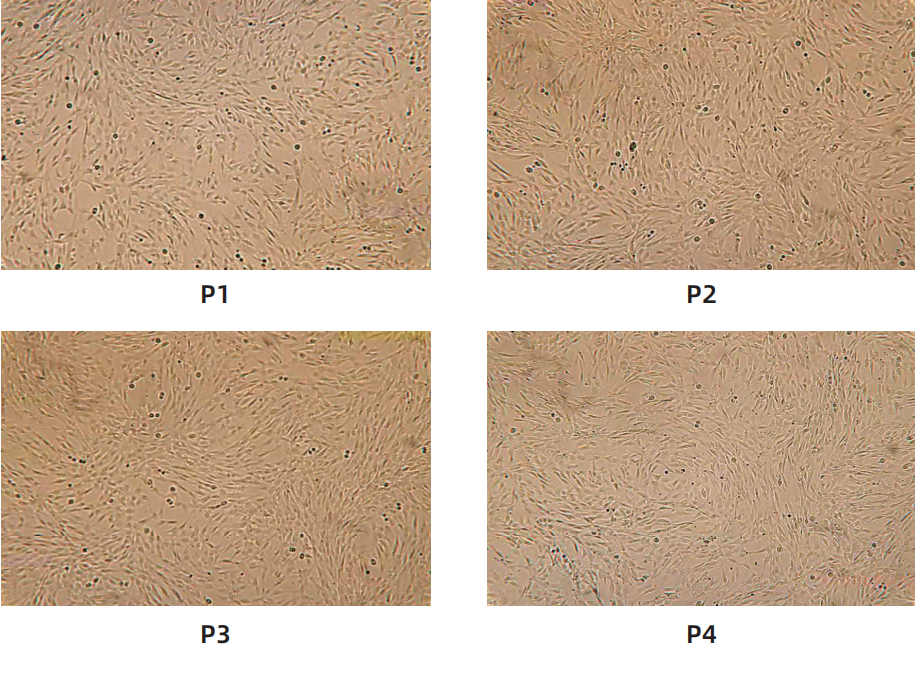
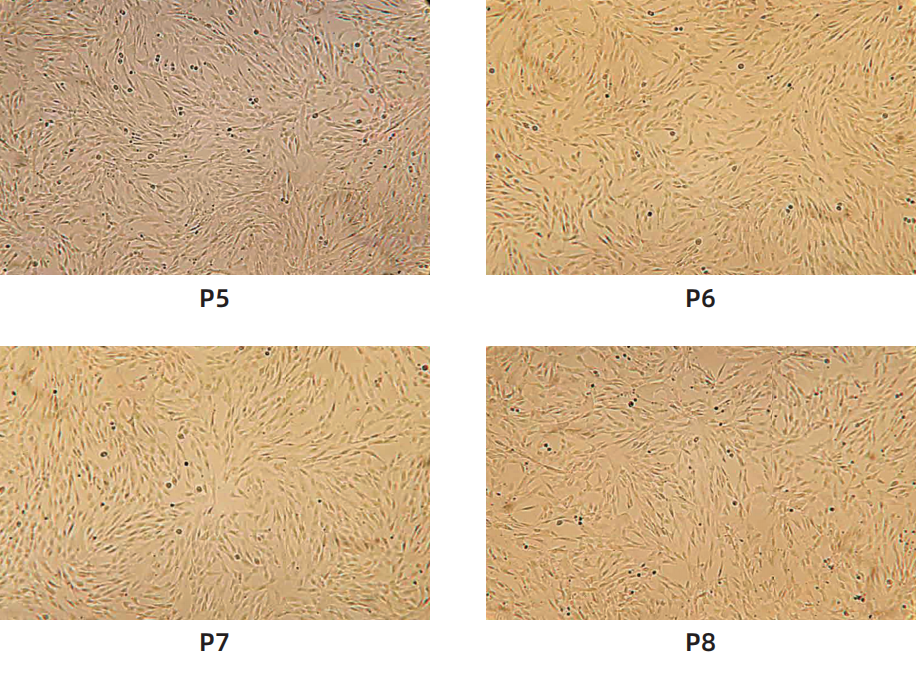
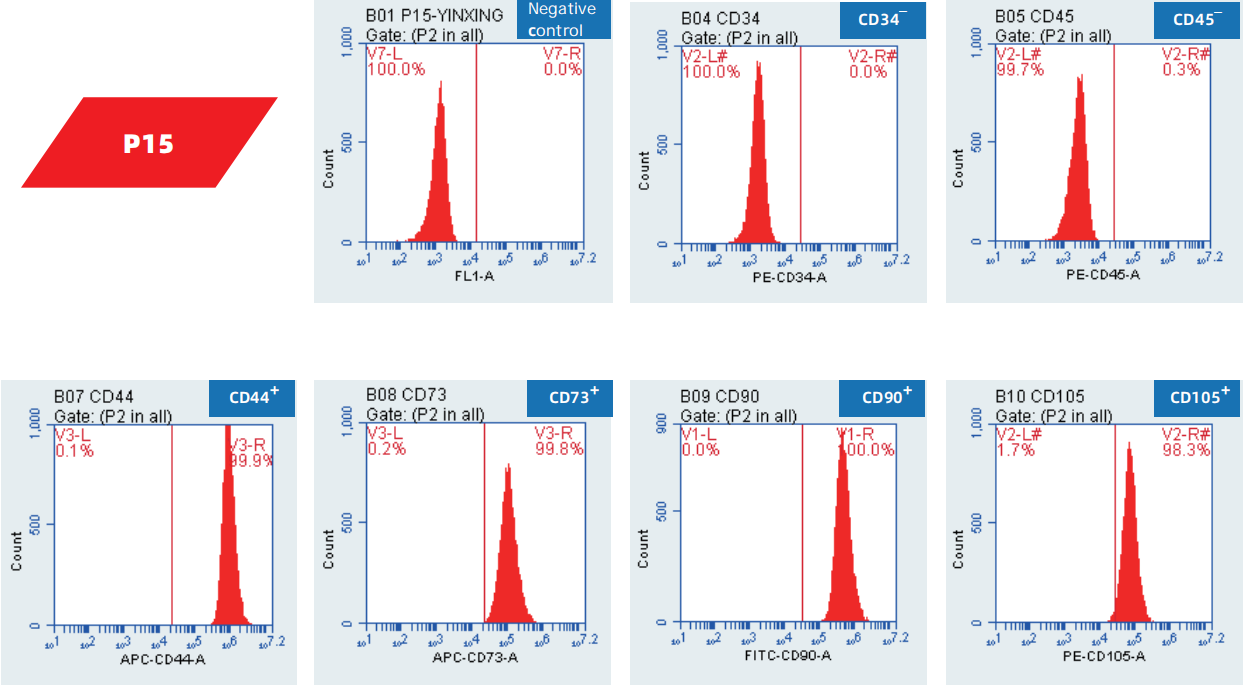
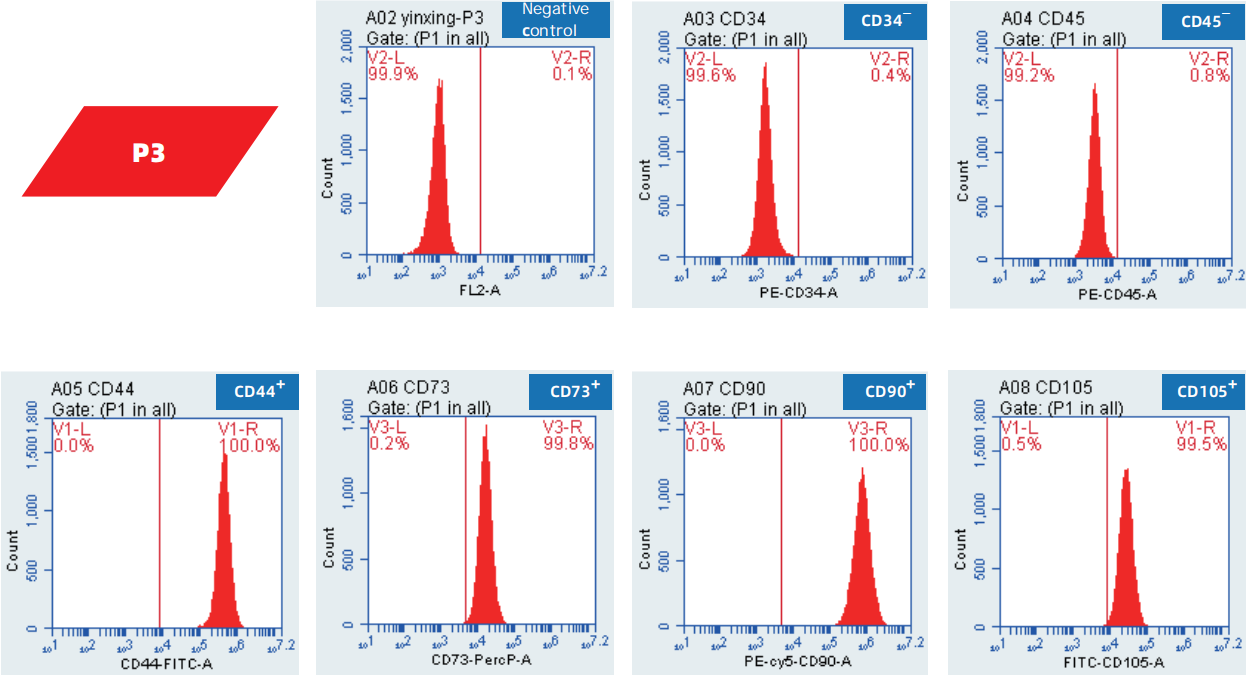
Surface marker identification
Approximately 2.4 × 10^7 primary cells can be obtained from a fresh 20cm umbilical cord.
Primary cells begin to migrate out within 5-9 days.
Primary cells can be harvested within 12-14 days.
Data on primordial cell isolation
A single umbilical cord can yield approximately 16cm of usable tissue, with each 150mm culture dish requiring about 2cm of cord. This allows for the cultivation of up to 8 dishes. On average, 150 such dishes (equivalent to a T175 culture flask) can produce between 1E6 and 3E6 primary cells, with each cord yielding 8E6-2.4E7 cells.

Primary cells isolated from the umbilical cord or cells from the established seed bank can be subcultured and stably passaged up to 20 generations.
Total yield of P3 cells: 6480 × 10^7 cells.
Total yield of P10 cells: 58032 × 10^13 cells.
Total yield of P20 cells: 424351 × 10^20 cells.
Data of Continuous Subculturing

*The above data was obtained from serum-free medium and stem cell mild digestive enzymes by Yocon Biology primary separation technique. Different umbilical cord samples and different separation techniques may lead to significant differences in results.
Cell morphology





Surface marker identification


True Serum-Free Formulation: Eliminates the need for animal serum, minimizing lot-to-lot variability and reducing the risk of contamination from animal-derived pathogens.
High Proliferation Capacity: Supports rapid and efficient expansion of MSCs, yielding a large number of high-quality cells.
Maintained Cell Characteristics: Ensures that MSCs retain their essential characteristics, including morphology, immunophenotype, and multipotent differentiation capabilities through extended passages.
Biocompatibility and Safety Tested: Rigorously tested for biocompatibility and toxicity to ensure the highest safety profile for cellular applications.
Regulatory Support: US FDA 510(K) clearance simplifies the regulatory pathway for downstream drug applications.
Enhanced Research Reliability: Achieve more consistent and reproducible results due to the defined and serum-free nature of the medium, reducing experimental variability.
Accelerated Development: The high cell yield and rapid expansion capabilities shorten the time required for research and development cycles.
Superior Safety Profile: Eliminate concerns about xeno-contamination, making this medium ideal for clinical and therapeutic applications where product safety is paramount.
True Serum-Free Formulation: Eliminates the need for animal serum, minimizing lot-to-lot variability and reducing the risk of contamination from animal-derived pathogens.
High Proliferation Capacity: Supports rapid and efficient expansion of MSCs, yielding a large number of high-quality cells.
Maintained Cell Characteristics: Ensures that MSCs retain their essential characteristics, including morphology, immunophenotype, and multipotent differentiation capabilities through extended passages.
Biocompatibility and Safety Tested: Rigorously tested for biocompatibility and toxicity to ensure the highest safety profile for cellular applications.
Regulatory Support: US FDA 510(K) clearance simplifies the regulatory pathway for downstream drug applications.
Enhanced Research Reliability: Achieve more consistent and reproducible results due to the defined and serum-free nature of the medium, reducing experimental variability.
Accelerated Development: The high cell yield and rapid expansion capabilities shorten the time required for research and development cycles.
Superior Safety Profile: Eliminate concerns about xeno-contamination, making this medium ideal for clinical and therapeutic applications where product safety is paramount.
Primary Isolation of MSCs: Optimized for the initial isolation of mesenchymal stem cells from various human tissues including umbilical cord, adipose tissue, bone marrow, and more.
Large-Scale MSC Expansion: Ideal for expanding MSC populations for research, clinical trials, and therapeutic manufacturing.
Stem Cell Research: Supports studies on MSC biology, differentiation, and regenerative medicine.
Drug Discovery and Development: Provides a reliable platform for high-throughput screening and development of cell-based therapies.
Primary Isolation of MSCs: Optimized for the initial isolation of mesenchymal stem cells from various human tissues including umbilical cord, adipose tissue, bone marrow, and more.
Large-Scale MSC Expansion: Ideal for expanding MSC populations for research, clinical trials, and therapeutic manufacturing.
Stem Cell Research: Supports studies on MSC biology, differentiation, and regenerative medicine.
Drug Discovery and Development: Provides a reliable platform for high-throughput screening and development of cell-based therapies.