English 


| Availability: | |
|---|---|
▶ greatly reduce the probability of pollution ▶ highly standardized ▶ highly automated
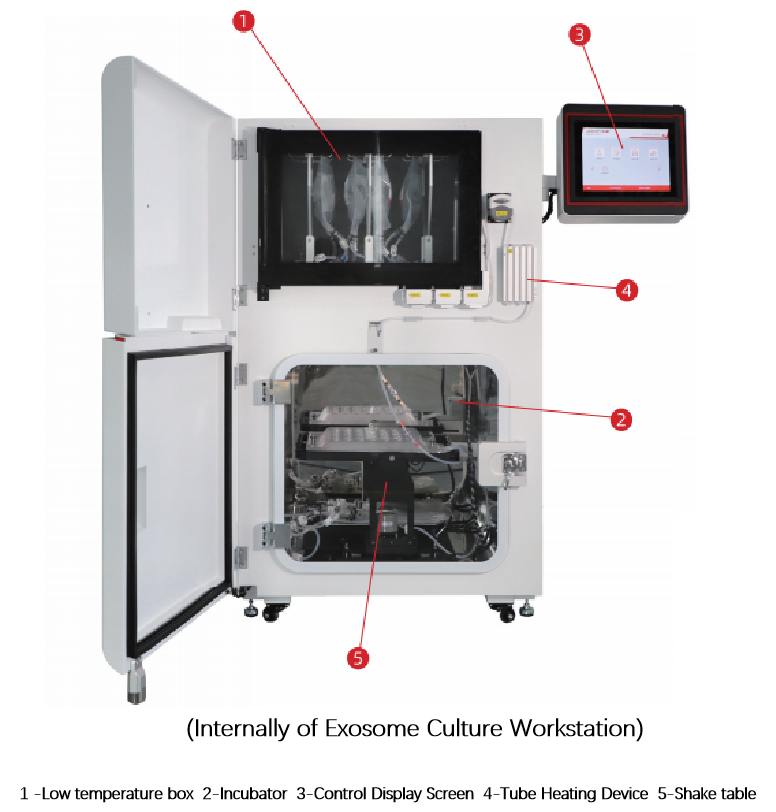
Exosome culture workstation is an intelligent device designed for large-scale and efficient automated production of mesenchymal stem cell exosomes. Through intermittent shaking, the cultivation and harvest of exosomes secreted by stem cells can be completed automatically.
This workstation integrates critical processes including cell culture amplification and exosome production. The entire cultivation cycle spans 15 days using 5L bagged culture medium, yielding 5L of exosome supernatant. It achieves full automation from cell processing to exosome extraction, significantly enhancing both production efficiency and exosome quality.
The system features highly intelligent design, requiring no manual intervention after cell seeding. Its sterile closed-loop pipeline system with FEP material contains pre-packaged sterile disc-shaped microcarriers that are ready-to-use without prior sterilization. The fully enclosed operation of the equipment and associated pipelines minimizes environmental requirements, ensuring safe cultivation outcomes while maintaining strict compliance with safety standards.
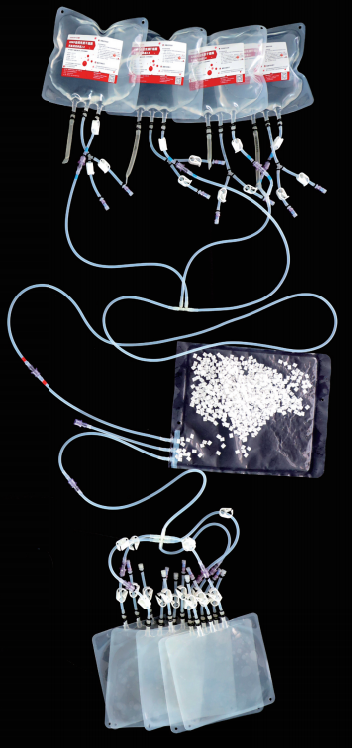
Product Structure
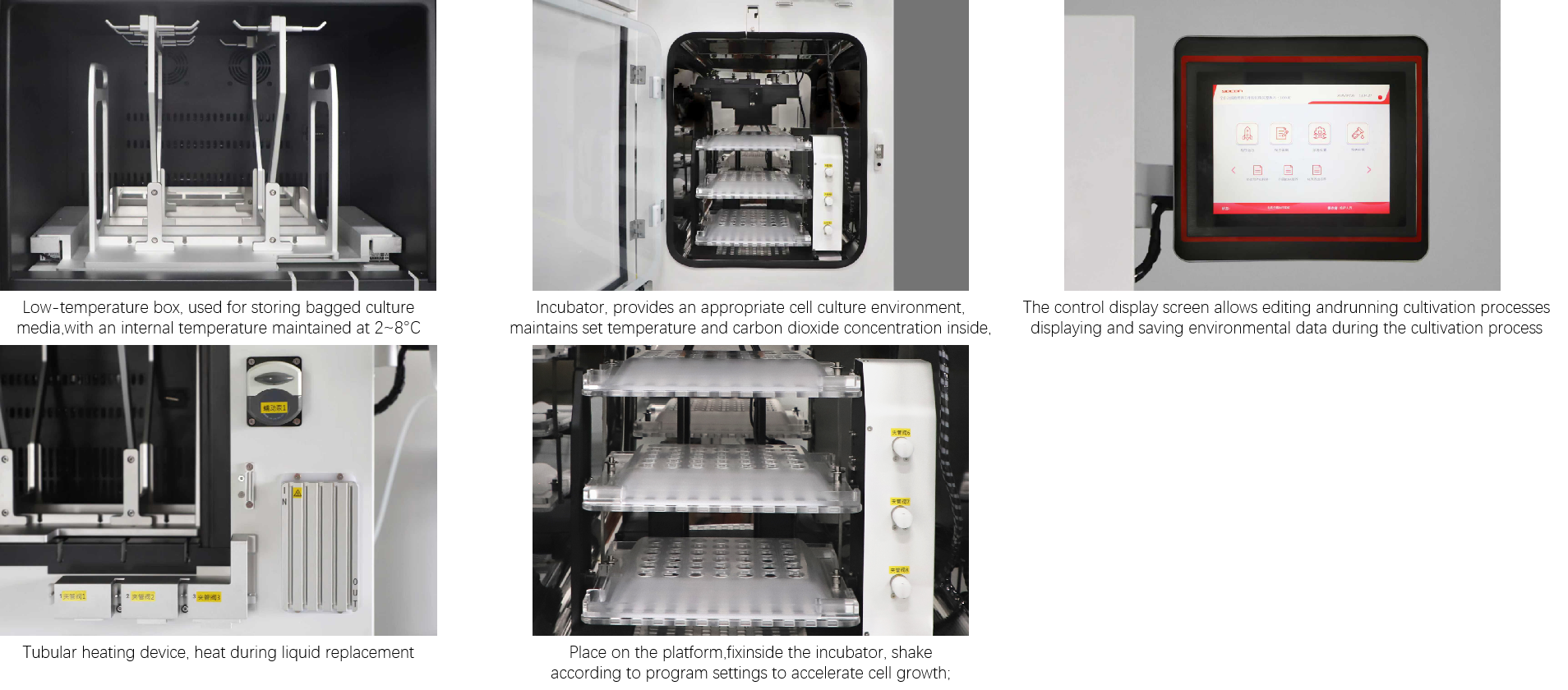
Comprehensive Supporting Technologies
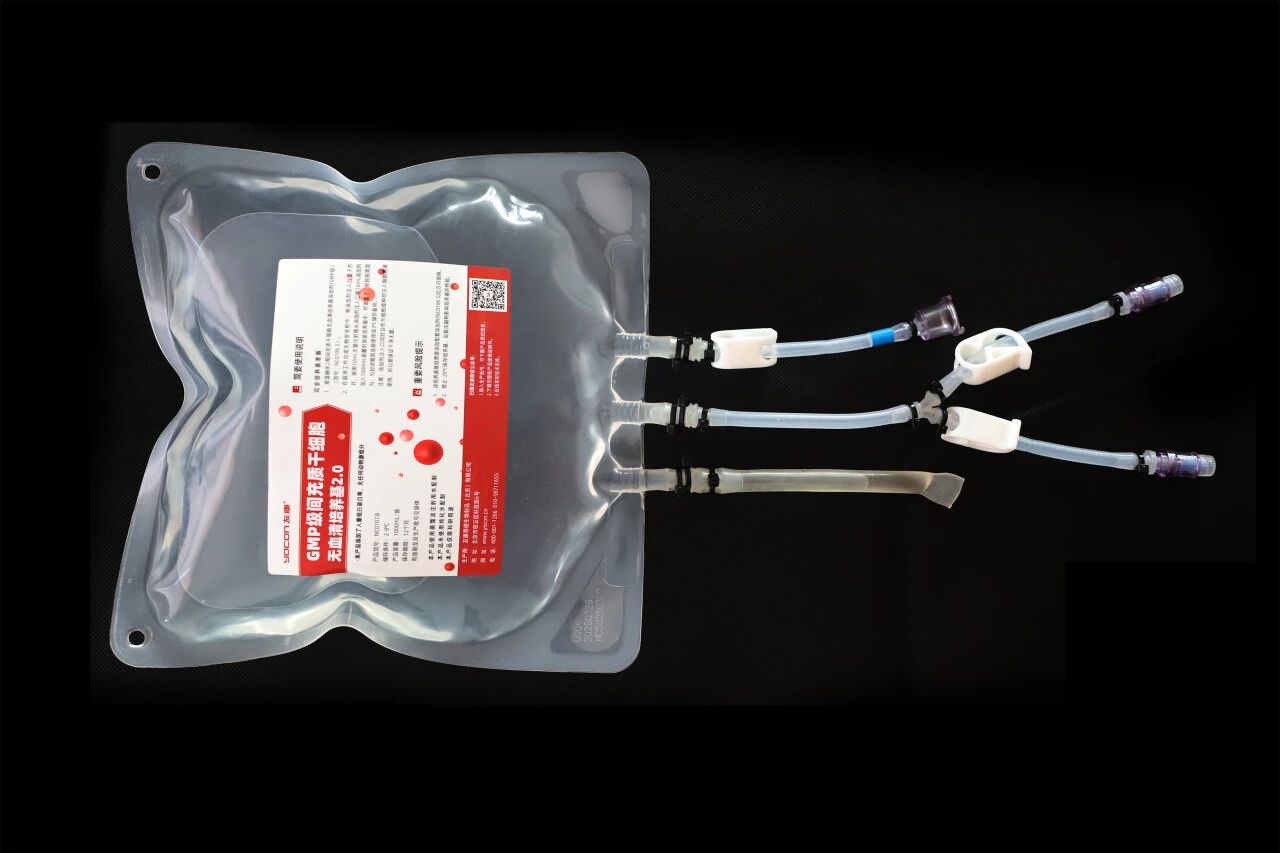
Technology 1: (Bagged Liquid Medium for Mesenchymal Stem Cells)
The exosome culture workstation enables the automated production of exosomes, relying on the bagged liquid medium. During the manufacturing process of this product, it is directly filled into a sterile bag. During use, it enters the cell culture bag through a fully enclosed pipeline system, without any contact with the outside environment throughout the process, ensuring that the medium remains sterile at all times. This packaging method effectively avoids external contamination and guarantees the safety of exosome production.
The bagged culture medium is packaged with multi - layer co - extruded film. This film has the advantages of high barrier properties, strong functionality, high strength, and no pollution, and it also exhibits excellent biocompatibility.

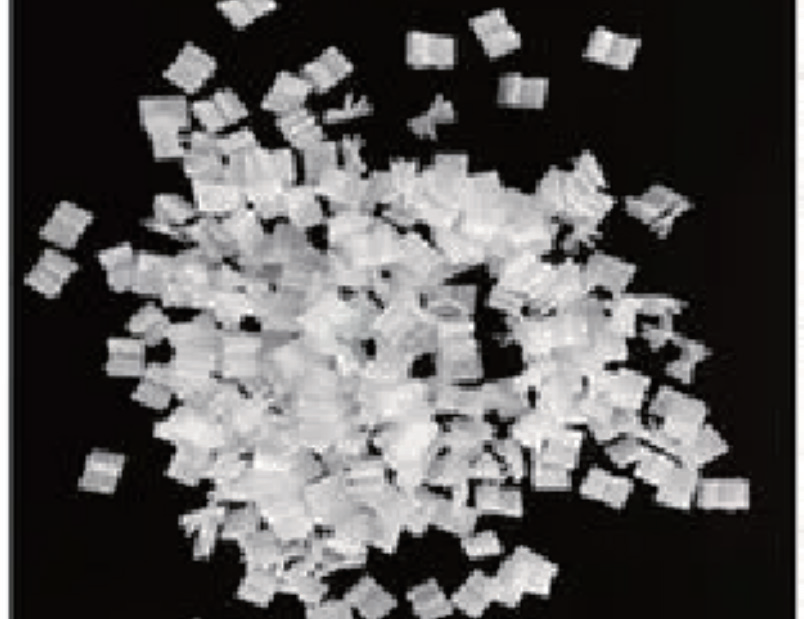
Technology 2: (Pre - packaged Sterile Flake - shaped Microcarriers)
The flake - shaped carrier is mainly made of medical - grade polymer polyester fiber (PET). Through electrostatic ultrasonic spinning technology, it is thermally bonded and processed into a non - woven fabric substrate with a porous structure. After further processes such as welding, shaping, cleaning, and surface modification, it is prepared into a flake - shaped solid growth - supporting matrix for adherent - dependent cell culture.
As the main material of the flake - shaped carrier, the 3D reticular three - dimensional structure fabric formed by PET has characteristics such as good acid - and alkali - resistance, heat - resistance, non - toxicity, and non - biodegradability. It has moderate stiffness and good flexibility, and can well tolerate high fluid stress. There are no additives during the processing, which is non - toxic and harmless to cell growth and meets the requirements of pharmaceutical manufacturing.
The flake - shaped carrier is a highly efficient micro - carrier specifically designed for adherent culture of mammalian cells. It has a single - layer thickness of 0.44 mm and a pore size of approximately 15 μm. It can provide sufficient surface area for cell growth, enabling cells to reproduce and grow in a three - dimensional structural space. At the same time, it maintains good exchange of various nutrients and reduces the accumulation of harmful metabolites, providing a favorable micro - environment for cell growth and achieving high - density cell culture.
Since the carrier has a certain cell - retaining effect, when obtaining the cell harvest solution, there is no need to use cell - retaining devices such as rotary filters or sedimentation columns. This reduces the content of cells and DNA in the harvest solution. Compared with other culture systems, it is more conducive to the downstream purification of exosome products.
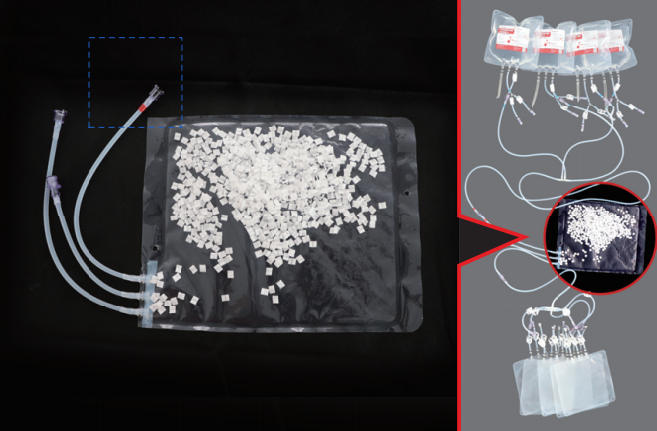
Technology 3: (Fully Enclosed Single - use Pipeline System)

FEP Membrane Material
FEP is short for perfluoroethylene propylene copolymer. It is a 100% inert material, a single - layer, high - purity substance. No additives or other biological source materials are added during the processing, showing excellent biocompatibility.
FEP has good oxygen permeability and water vapor barrier properties, which can meet the gas exchange required during cell growth. Under the same culture volume, a higher number of cells can be obtained.
FEP has a light transmittance of over 95%, allowing for a very intuitive observation of the cells inside the bag. This enables a better assessment of the cell growth state and ensures the safety of the cells.
Silicone Tube
Medical - grade silicone tubes are used. The material has passed relevant biocompatibility tests and exhibits good chemical stability, meeting the requirements of high - temperature sterilization and irradiation sterilization.
Injection - molded Parts
All injection - molded parts are made of medical - grade raw materials, which have good biocompatibility, providing good connection stability and safety for the entire system.
▶ greatly reduce the probability of pollution ▶ highly standardized ▶ highly automated
Exosome culture workstation is an intelligent device designed for large-scale and efficient automated production of mesenchymal stem cell exosomes. Through intermittent shaking, the cultivation and harvest of exosomes secreted by stem cells can be completed automatically.
This workstation integrates critical processes including cell culture amplification and exosome production. The entire cultivation cycle spans 15 days using 5L bagged culture medium, yielding 5L of exosome supernatant. It achieves full automation from cell processing to exosome extraction, significantly enhancing both production efficiency and exosome quality.
The system features highly intelligent design, requiring no manual intervention after cell seeding. Its sterile closed-loop pipeline system with FEP material contains pre-packaged sterile disc-shaped microcarriers that are ready-to-use without prior sterilization. The fully enclosed operation of the equipment and associated pipelines minimizes environmental requirements, ensuring safe cultivation outcomes while maintaining strict compliance with safety standards.
Product Structure


Comprehensive Supporting Technologies
Technology 1: (Bagged Liquid Medium for Mesenchymal Stem Cells)
The exosome culture workstation enables the automated production of exosomes, relying on the bagged liquid medium. During the manufacturing process of this product, it is directly filled into a sterile bag. During use, it enters the cell culture bag through a fully enclosed pipeline system, without any contact with the outside environment throughout the process, ensuring that the medium remains sterile at all times. This packaging method effectively avoids external contamination and guarantees the safety of exosome production.
The bagged culture medium is packaged with multi - layer co - extruded film. This film has the advantages of high barrier properties, strong functionality, high strength, and no pollution, and it also exhibits excellent biocompatibility.


Technology 2: (Pre - packaged Sterile Flake - shaped Microcarriers)
The flake - shaped carrier is mainly made of medical - grade polymer polyester fiber (PET). Through electrostatic ultrasonic spinning technology, it is thermally bonded and processed into a non - woven fabric substrate with a porous structure. After further processes such as welding, shaping, cleaning, and surface modification, it is prepared into a flake - shaped solid growth - supporting matrix for adherent - dependent cell culture.
As the main material of the flake - shaped carrier, the 3D reticular three - dimensional structure fabric formed by PET has characteristics such as good acid - and alkali - resistance, heat - resistance, non - toxicity, and non - biodegradability. It has moderate stiffness and good flexibility, and can well tolerate high fluid stress. There are no additives during the processing, which is non - toxic and harmless to cell growth and meets the requirements of pharmaceutical manufacturing.
The flake - shaped carrier is a highly efficient micro - carrier specifically designed for adherent culture of mammalian cells. It has a single - layer thickness of 0.44 mm and a pore size of approximately 15 μm. It can provide sufficient surface area for cell growth, enabling cells to reproduce and grow in a three - dimensional structural space. At the same time, it maintains good exchange of various nutrients and reduces the accumulation of harmful metabolites, providing a favorable micro - environment for cell growth and achieving high - density cell culture.
Since the carrier has a certain cell - retaining effect, when obtaining the cell harvest solution, there is no need to use cell - retaining devices such as rotary filters or sedimentation columns. This reduces the content of cells and DNA in the harvest solution. Compared with other culture systems, it is more conducive to the downstream purification of exosome products.


Technology 3: (Fully Enclosed Single - use Pipeline System)


FEP Membrane Material
FEP is short for perfluoroethylene propylene copolymer. It is a 100% inert material, a single - layer, high - purity substance. No additives or other biological source materials are added during the processing, showing excellent biocompatibility.
FEP has good oxygen permeability and water vapor barrier properties, which can meet the gas exchange required during cell growth. Under the same culture volume, a higher number of cells can be obtained.
FEP has a light transmittance of over 95%, allowing for a very intuitive observation of the cells inside the bag. This enables a better assessment of the cell growth state and ensures the safety of the cells.
Silicone Tube
Medical - grade silicone tubes are used. The material has passed relevant biocompatibility tests and exhibits good chemical stability, meeting the requirements of high - temperature sterilization and irradiation sterilization.
Injection - molded Parts
All injection - molded parts are made of medical - grade raw materials, which have good biocompatibility, providing good connection stability and safety for the entire system.
Advanced Automated Culture and Harvesting
The system employs a sophisticated intermittent oscillation mechanism that significantly enhances cell growth and exosome release. This dynamic process ensures optimal nutrient delivery and waste removal, leading to higher exosome yields and improved consistency compared to static culture methods.
Fully Integrated Workflow
Say goodbye to fragmented processes. This workstation seamlessly integrates all critical steps, from initial cell seeding and expansion to continuous exosome production and automated harvesting. This holistic approach reduces the need for multiple pieces of equipment and minimizes transfer steps, thereby lowering contamination risks.
Exceptional High Yield Capacity
Engineered for scalability, the system is capable of efficiently producing up to 5 liters of exosome-containing supernatant from just 5 liters of culture medium. This impressive output is crucial for applications requiring large quantities of exosomes, such as preclinical studies or large-scale therapeutic development.
True Intelligent Automation (Set-and-Forget)
Once your cells are seeded, the workstation handles the rest. Our intelligent control system precisely manages all parameters—temperature, CO2 levels, oscillation speed, and harvesting schedules—with zero manual intervention required. This drastically reduces labor costs, frees up valuable personnel time, and eliminates human error.
State-of-the-Art Sterile Closed System
Safety and purity are paramount. The workstation utilizes a completely closed pipeline system constructed from high-purity FEP material, known for its inertness and excellent biocompatibility. It also incorporates pre-packaged, ready-to-use sterile flake-shaped microcarriers, minimizing exposure to the external environment and ensuring an aseptic culture condition throughout the entire process.
Product Advantages
Automation
Equipped with a disposable fully - enclosed pipeline system with pre - packaged flake - shaped microcarriers, which can be used immediately upon retrieval. The entire process from cell culture to exosome production is automated. This reduces requirements for the external environment and saves costs.
Scalability
The system automatically records all operation steps in the whole process, providing more precise and consistent culture conditions. It offers a more intelligent and scalable culture solution for exosome production in small - and medium - sized systems.
Standardization
It provides a mature and complete production process with standardized production procedures. A set of standardized production processes can be quickly replicated in multiple laboratories of users, helping users rapidly establish their own product systems.
Handmade culture | Traditional methods |
| It involves 20 to 30 subdivisions, each of which requires manual operation |
bioreactor | high-quality high cost |
| The time required for cleaning and sterilization depends on On the type, size and sterilization of the reactor The method usually takes 6 to 24 hours |
exosome Training station | High quality, low cost |
| It takes only two hours to get on the plane |
Advanced Automated Culture and Harvesting
The system employs a sophisticated intermittent oscillation mechanism that significantly enhances cell growth and exosome release. This dynamic process ensures optimal nutrient delivery and waste removal, leading to higher exosome yields and improved consistency compared to static culture methods.
Fully Integrated Workflow
Say goodbye to fragmented processes. This workstation seamlessly integrates all critical steps, from initial cell seeding and expansion to continuous exosome production and automated harvesting. This holistic approach reduces the need for multiple pieces of equipment and minimizes transfer steps, thereby lowering contamination risks.
Exceptional High Yield Capacity
Engineered for scalability, the system is capable of efficiently producing up to 5 liters of exosome-containing supernatant from just 5 liters of culture medium. This impressive output is crucial for applications requiring large quantities of exosomes, such as preclinical studies or large-scale therapeutic development.
True Intelligent Automation (Set-and-Forget)
Once your cells are seeded, the workstation handles the rest. Our intelligent control system precisely manages all parameters—temperature, CO2 levels, oscillation speed, and harvesting schedules—with zero manual intervention required. This drastically reduces labor costs, frees up valuable personnel time, and eliminates human error.
State-of-the-Art Sterile Closed System
Safety and purity are paramount. The workstation utilizes a completely closed pipeline system constructed from high-purity FEP material, known for its inertness and excellent biocompatibility. It also incorporates pre-packaged, ready-to-use sterile flake-shaped microcarriers, minimizing exposure to the external environment and ensuring an aseptic culture condition throughout the entire process.
Product Advantages
Automation
Equipped with a disposable fully - enclosed pipeline system with pre - packaged flake - shaped microcarriers, which can be used immediately upon retrieval. The entire process from cell culture to exosome production is automated. This reduces requirements for the external environment and saves costs.
Scalability
The system automatically records all operation steps in the whole process, providing more precise and consistent culture conditions. It offers a more intelligent and scalable culture solution for exosome production in small - and medium - sized systems.
Standardization
It provides a mature and complete production process with standardized production procedures. A set of standardized production processes can be quickly replicated in multiple laboratories of users, helping users rapidly establish their own product systems.
Handmade culture | Traditional methods |
| It involves 20 to 30 subdivisions, each of which requires manual operation |
bioreactor | high-quality high cost |
| The time required for cleaning and sterilization depends on On the type, size and sterilization of the reactor The method usually takes 6 to 24 hours |
exosome Training station | High quality, low cost |
| It takes only two hours to get on the plane |
1、Exosome concentration, particle size
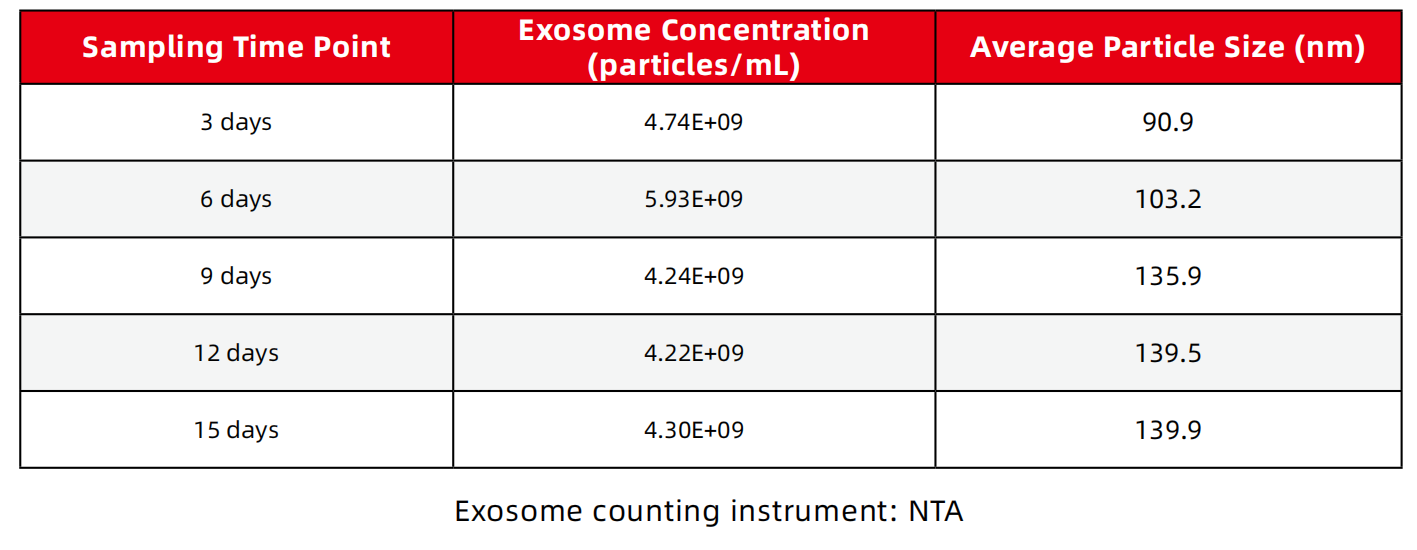
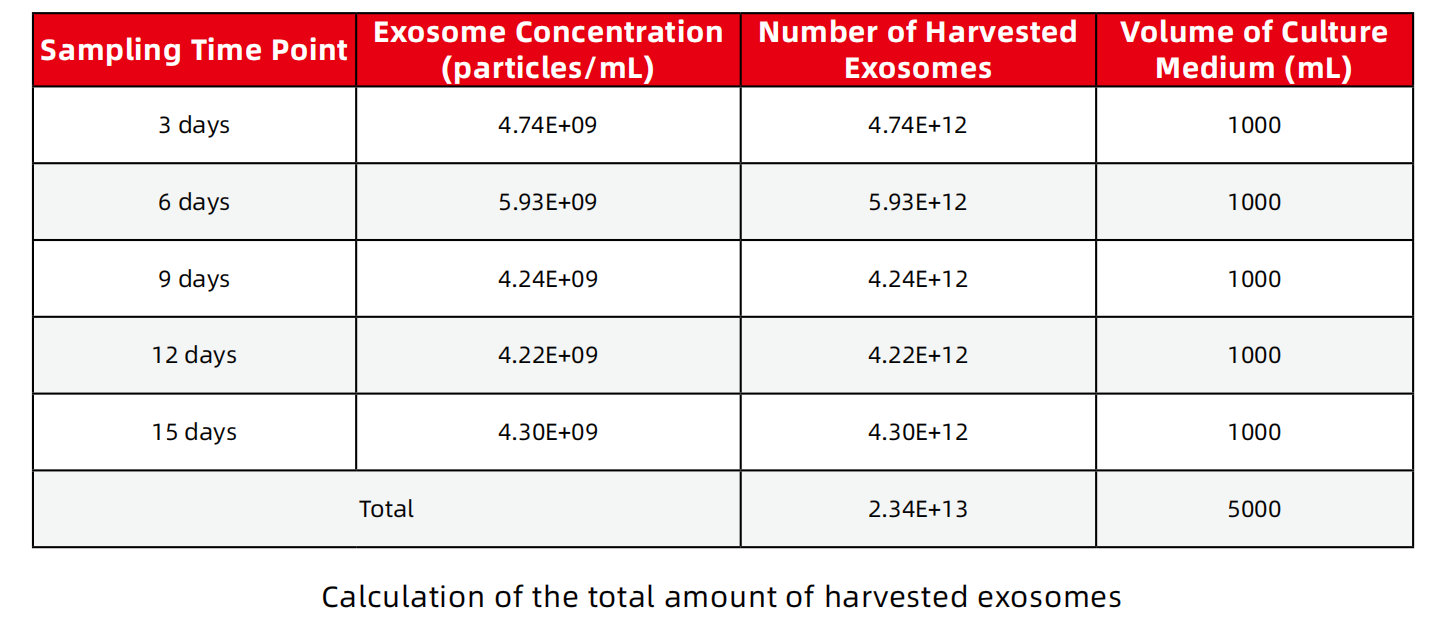
2、The total amount of harvested exosomes
A cultivation cycle is 15 days, requiring 5L of culture medium. There is a cycle every 3 days, and 1L of supernatant can be harvested. The total number of harvested exosomes is 2.34E+13.
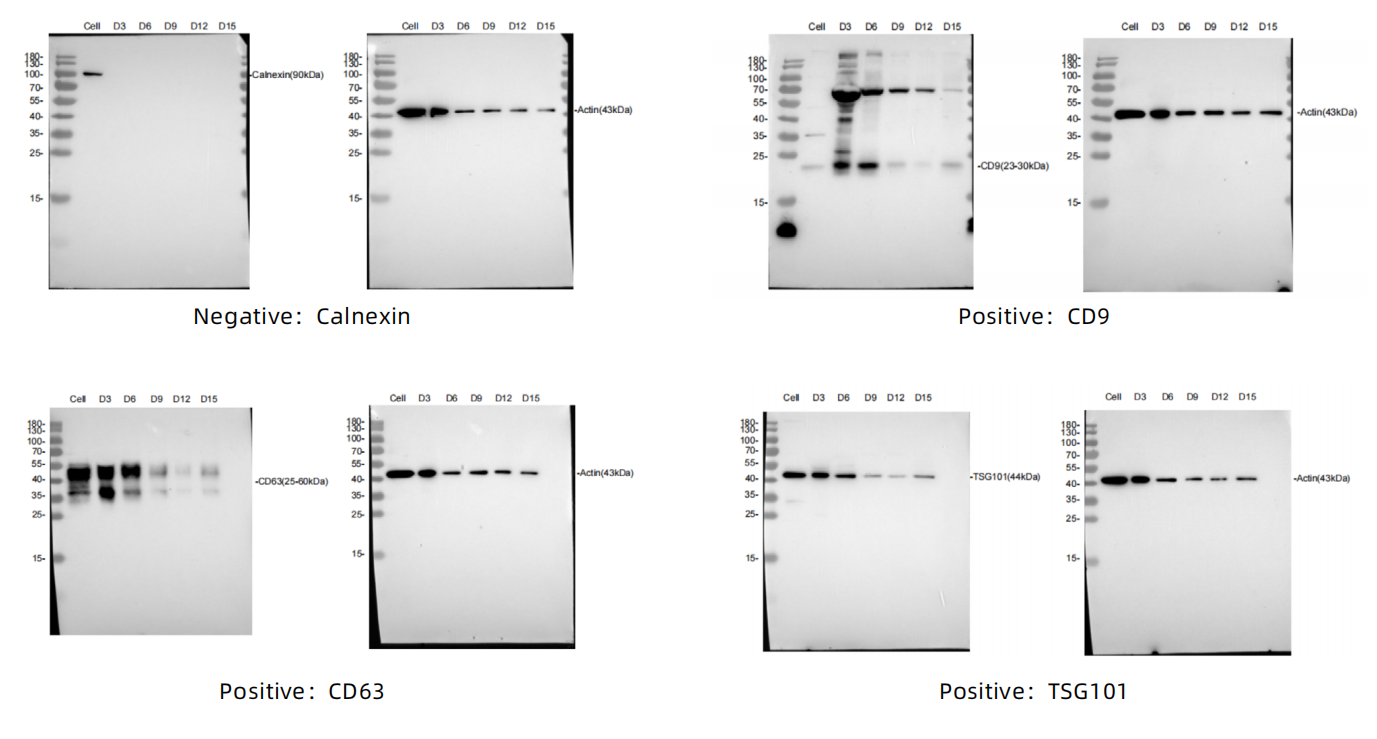
3、Exosome - marker proteins
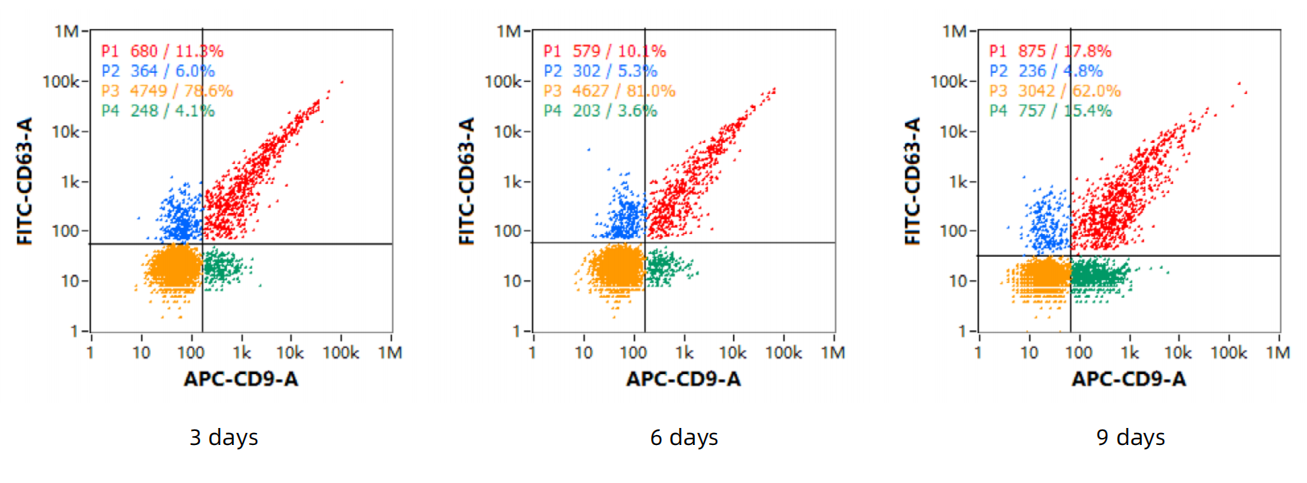
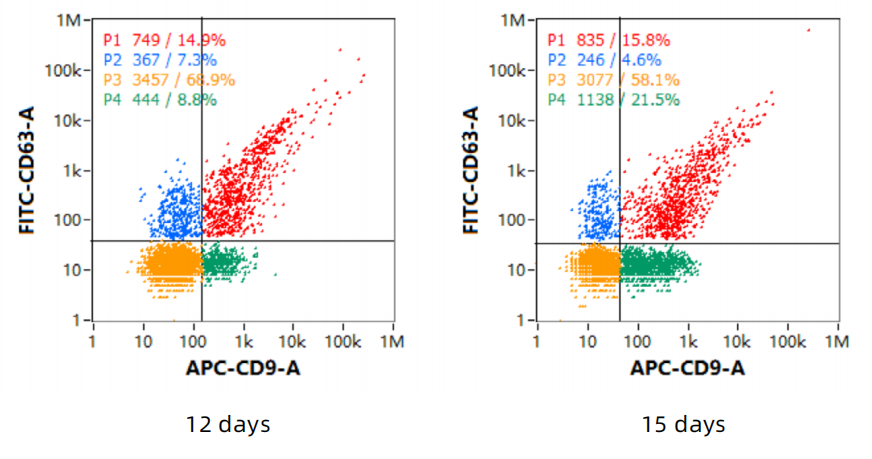
4、Detect the protein markers by Western - Blot (WB)
Negative: Calnexin
Positive: CD9, TSG101, CD63
1、Exosome concentration, particle size

2、The total amount of harvested exosomes
A cultivation cycle is 15 days, requiring 5L of culture medium. There is a cycle every 3 days, and 1L of supernatant can be harvested. The total number of harvested exosomes is 2.34E+13.

3、Exosome - marker proteins


4、Detect the protein markers by Western - Blot (WB)
Negative: Calnexin
Positive: CD9, TSG101, CD63

Therapeutic Exosome Development
Crucial for companies and research institutions developing exosome-based therapies for various diseases, including regenerative medicine, immune modulation, and targeted drug delivery. The workstation provides the consistent, large-scale output needed for preclinical and clinical trials.
Biomarker Discovery and Diagnostics
Supports the isolation of exosomes for biomarker research, where consistent exosome quality and quantity are vital for identifying disease-specific markers in liquid biopsies and developing novel diagnostic tools.
Fundamental Exosome Research
Accelerates basic science investigations into exosome biology, their roles in intercellular communication, and their potential as delivery vehicles. Researchers can reliably obtain sufficient exosomes for in-depth proteomic, transcriptomic, and lipidomic analyses.
Therapeutic Exosome Development
Crucial for companies and research institutions developing exosome-based therapies for various diseases, including regenerative medicine, immune modulation, and targeted drug delivery. The workstation provides the consistent, large-scale output needed for preclinical and clinical trials.
Biomarker Discovery and Diagnostics
Supports the isolation of exosomes for biomarker research, where consistent exosome quality and quantity are vital for identifying disease-specific markers in liquid biopsies and developing novel diagnostic tools.
Fundamental Exosome Research
Accelerates basic science investigations into exosome biology, their roles in intercellular communication, and their potential as delivery vehicles. Researchers can reliably obtain sufficient exosomes for in-depth proteomic, transcriptomic, and lipidomic analyses.